Ruolo degli anticorpi monoclonali in una strategia di cura
Re: Ruolo degli anticorpi monoclonali in una strategia di cura
Ciao Dora, il micio sta bene (più o meno) anche senza le costose iniezioni mensili, i gatti sopportano il dolore molto più di noi umani, e cerco di accudirlo nel miglior modo possibile. Come hai scritto, la mania degli anticorpi è dilagata (forse questo mi ha reso riluttante) e non voglio spendere una fortuna, come nel caso di cure omeopatiche. Gli antidolorifici possono essere assunti solo per breve periodo, quindi, poverello, lo lascio così. (D'altronde anche io ho un mal di schiena cronico e non posso inghiottire brufen tutti i giorni). La frase "anticorpi monoclonali" l'ho appresa durante il periodo Covid e ora si legge dappertutto, da qui i miei dubbi. Ben vengano se hanno la capacità di neutralizzare il virus (da ciò che ho capito).
Re: Ruolo degli anticorpi monoclonali in una strategia di cura
Questo fact sheet appena pubblicato da TAG è uno strumento molto utile per sintetizzare le ricerche sugli anticorpi ad amplissimo spettro per una cura dell'infezione da HIV:
Broadly Neutralizing Antibodies (bNAbs) and HIV Cure-Related Research
Broadly Neutralizing Antibodies (bNAbs) and HIV Cure-Related Research
Re: Ruolo degli anticorpi monoclonali in una strategia di cura
Dal CROI:
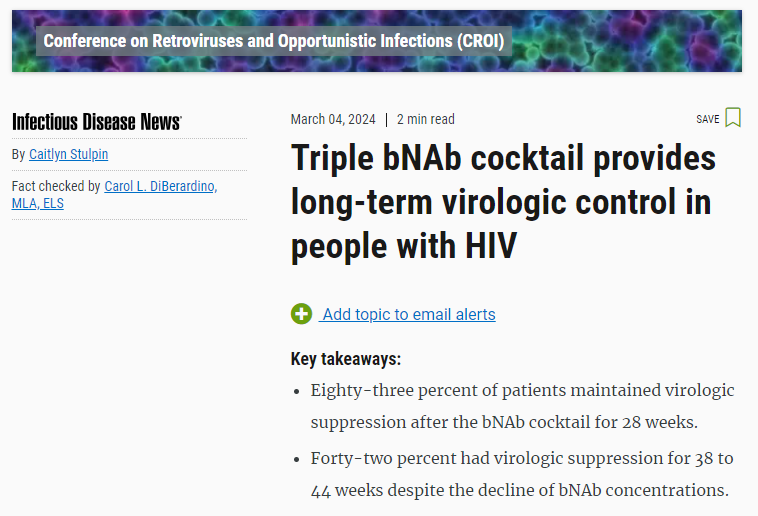

Triple bNAb cocktail provides long-term virologic control in people with HIV
DENVER — A cocktail composed of three broadly neutralizing monoclonal antibodies resulted in long-term virologic control in people with HIV after they discontinued ART, researchers found.
“Broadly neutralizing antibodies have been around for about a decade now and are being developed for prevention but also for therapy. These antibodies have the ability to recognize a large variety of HIV strains that have been identified in individuals with chronic infection,” Boris D. Juelg, MD, PhD, principal investigator at the Ragon Institute of Mass General, MIT, and Harvard. Infectious Diseases Division, said at a press conference held during the Conference on Retroviruses and Opportunistic Infections.
Data derived from Juelg BD, et al. Abstract 121. Presented at: Conference on Retroviruses and Opportunistic Infections; March 3-6, 2024; Denver.
“The issue is that there's viral escape and a selection of resistant viral variants, which is an obstacle for that therapy,” he said. “So the idea then was to have more antibodies and increase the breadth of neutralization potency, by using antibodies that target different aspects of the virus.”
For the study, Juelg and colleagues first evaluated the pharmacokinetics of the broadly neutralizing monoclonal antibodies (bNAbs) PGT121, which targets the V3 glyvan supersite; PGDM1400, which targets the V2 apex; and VRC07-523LS, which targets the CD4 binding site.
The researchers then assessed the therapeutic efficacy of up to six monthly infusions of this triple bNAb cocktail in 12 people with HIV who discontinued ART after the first infusion of the cocktail.
Overall, 83% of participants (n = 10) maintained virologic suppression for the duration of the antibody dosing period for at least 28 weeks, whereas 42% of participants (n = 5) demonstrated virologic suppression for the duration of follow-up for at least 38 to 44 weeks, despite the decline of serum bNAb concentrations to low or undetectable levels.
Additional data from the study showed early viral rebound in two patients, which correlated with baseline resistance to two of the bNAbs — PGT121 and PGDM1400 — whereas late viral rebound in five participants in the context of declining bNAb levels was characterized by sensitive and resistant rebound virus.
“Overall, this study showed that the three antibodies with a significant breadth of neutralization were actually able to maintain suppression at least during the dosing period in a large majority of the participants, and in a smaller subset, this control was maintained when the antibodies had reached very low levels up to week 44,” Juegl said. “Future studies have to show that is maintained for much longer and what the exact mechanisms of control are.”
“This is something that we're working on right now,” he said.
Source: Juelg BD, et al. Abstract 121. Presented at: Conference on Retroviruses and Opportunistic Infections; March 3-6, 2024; Denver.
Disclosures: The authors report no relevant financial disclosures.
Re: Ruolo degli anticorpi monoclonali in una strategia di cura
L'articolo relativo al post del 2021, uscito sul New England Journal of Medicine: Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 AcquisitionDora ha scritto: ↑mercoledì 27 gennaio 2021, 7:38
Immunizzazione passiva con VRC01 - i risultati dello studio AMP (Antibody Mediated Prevention)
Alla 4th International HIV Research for Prevention Conference (HIVR4P) che si sta tenendo virtualmente in questi giorni, i ricercatori del National Institute of Allergy and Infectious Diseases (NIAID), in collaborazione con l'HIV Vaccine Trials Network (HVTN) e l'HIV Prevention Trials Network (HPTN), hanno presentato i risultati dei primi studi sull'immunizzazione passiva con un bNAb, VRC01.
Iniziato nel 2016, quando il primo bNAB isolato dal NIAID nel 2010 è stato reso disponibile per sperimentazioni sull'uomo, AMP si è svolto in due differenti trial, HVTN 704/HPTN 085 e HVTN 703/HPTN 081.
L'obiettivo era indagare se l'infusione di questo anticorpo era sicura e ben tollerata e se era in grado di proteggere dall'infezione da HIV persone ad alto rischio di infettarsi: circa 1900 donne reclutate in 7 Paesi dell'Africa Sub-sahariana (Botswana, Kenya, Malawi, Mozambico, Sud Africa, Tanzania, e Zimbabwe: dove prevale HIV-1 di sottotipo C) e circa 2400 MSM e donne transgender in Sud America (Brasile e Perù), Stati Uniti e Svizzera (dove a prevalere è l'HIV-1 di sottotipo B).
Metà dei partecipanti ha ricevuto una infusione dell'anticorpo, l'altra metà un placebo, ogni 8 settimane per 20 mesi, in tutto quindi 10 infusioni in un arco di tempo di 80 settimane (i risultati definitivi arriveranno quest'anno, i dati presentati oggi sono ancora parziali, ma alla fine le cose non cambieranno).
Due i dosaggi testati: 10 mg/kg e 30 mg/kg.
I partecipanti sono stati testati ogni 4 settimane e, se risultavano aver contratto l'infezione, il virus veniva analizzato per vedere se era suscettibile all'anticorpo.
La PrEP è stata offerta uniformemente in entrambe le sperimentazioni.
E vediamo come sono andate le cose.
Anzitutto, come già sappiamo dai diversi studi sulla cura fatti con questo anticorpo, VRC01 è stato sicuro e ben tollerato, senza effetti avversi di rilievo.
La protezione che ha offerto dall'infezione, però, è stata deludente, perché non è stata statisticamente migliore del placebo né nel dosaggio più basso, né in quello più alto.
Quello che è emerso dai due trial è che questo bNAb in monoterapia funziona solo nelle persone che non si imbattono in un HIV che è già di suo resistente a VRC01 e che solo il 30% dei virus circolanti nei Paesi dove si è svolto lo studio sono suscettibili a questo anticorpo (e negli studi sulla cura avevamo visto con quanta facilità il virus diventi resistente a VRC01 e in genere ai bNAbs).
Le conclusioni dei ricercatori sono state che - come già abbiamo visto dai trial sulla cura - una immunizzazione con un solo anticorpo non va bene. Saranno dunque necessari studi su combinazioni di bNAbs per vedere se si ottiene una buona protezione - e sappiamo che ce ne sono di molto più potenti di VRC01.
Commento di Jon Cohen:
E sua conclusione, che condivido:It has taken more than 4 years and $119 million for HIV researchers to test whether giving people infusions of antibodies made in a lab can protect them from the AIDS virus. Now, the unsatisfying answer is in: sometimes.
È vero che, finché non c'è un vaccino, tutto (o quasi) ci può andar bene, ma il ruolo che i bNAbs possono avere nella prevenzione di HIV è ancora tutto da stabilire e la competizione con la PrEP è fortissima.A powerful biomedical HIV prevention strategy already exists, however: a daily antiviral pill known as pre-exposure prophylaxis, or PrEP, which some AMP participants took as well. Monoclonal antibodies would have little appeal if PrEP was widely and consistently used, but many people have difficulty or don’t like taking daily pills, and its efficacy in women may be lower than men. Injected, long-acting antiretrovirals have also worked as PrEP in clinical studies but have yet to be licensed; they might provide a cheaper alternative to antibody infusions.
FONTI:
- comunicato stampa del NIAID: Antibody infusions prevent acquisition of some HIV strains, NIH studies find
comunicato stampa dell'HPTN: Most advanced clinical trials testing broadly neutralizing antibody against HIV demonstrate efficacy against sensitive strains
Jon Cohen su ScienceMag: Labmade antibodies can prevent HIV infection—but only if they match the virus
E veniamo all'oggi, quando la riflessione sull'uso degli anticorpi monoclonali si è approfondita grazie all'esperienza della pandemia di Covid: un uso massiccio di questi anticorpi - nei pochi Paesi dove è stato possibile, perché continuano ad essere costosi e di non facile somministrazione - si è accompagnato alla nascita e alla diffusione di varianti virali resistenti agli anticorpi stessi. Suona come già sentito? Proprio così: è una cosa che scriviamo da anni, ma l'esperienza del Covid l'ha resa inequivocabile.
E allora, che fare?
Prova a rispondere questa riflessione sullo IAVI Report:
Rethinking antibodies for infectious diseases?
The COVID-19 experience, along with lingering manufacturing and delivery concerns, is prompting a harder look at the future of antibody-based treatment and prevention for infectious diseases.
April 25, 2024
Michael Dumiak
The COVID-19 pandemic was the first time monoclonal antibody-based therapies were produced in mass quantities to combat an infectious disease. Clinics administered hundreds of thousands of antibody infusions and injections over the first two years of the pandemic with the aim of staving off the worst outcomes of SARS-CoV-2 infection.
It worked — at least in wealthier countries where these products were available — until it didn’t. The virus’s rapid evolution meant the antibodies were quickly outpaced.
This experience, along with other issues, has researchers assessing the future of antibody therapies for treating or preventing infectious diseases, including some of the most complicated pathogens such as HIV and antibiotic-resistant bacteria. “We are in the position that if you want more antibodies for infectious disease, you need to be very cautious,” says Rino Rappuoli, scientific director of the Biotecnopolo di Siena Foundation in Italy.
During the pandemic Rappuoli was chief scientist and head of external research and development at GSK vaccines, where he was instrumental in producing some of the first monoclonal antibody therapies against SARS-CoV-2. Today he and his colleagues within the Monoclonal Antibody Discovery Laboratory — the MAD Lab — continue their pursuit of new protein therapies as part of the Siena-based Fondazione Toscana Life Sciences center. But, as they do, the lessons from the COVID-19 pandemic are close to mind.
“We know they are safe and effective. But they have limitations,” Rappuoli says of monoclonal antibodies. “Because viruses can escape.”
Antibody therapies are a huge and growing field of biomedical research, but until COVID-19, their use has been mostly limited to cancer, immunotherapy, autoimmune conditions, and inflammation. Monoclonal antibodies are made by first isolating and then cloning the genes encoding the proteins that show effectiveness against a given pathogen: either by preventing or eliminating infection, or by reducing the risk or severity of symptoms. In recent years it has become possible to rapidly analyze thousands of proteins at a time.
This was, in part, why a monoclonal antibody against SARS-CoV-2 was the first therapeutic to receive an emergency use authorization from the U.S. Food and Drug Administration. By August 2021, in the grip of the Delta wave, the U.S. government was underwriting the shipment of 170,000 doses a week of an antibody therapy REGEN-COV, manufactured by Regeneron. Another 1.7 million doses of GSK’s and Vir Biotechnology’s sotrovimab, marketed under the trade name Xevudy, were also on order worldwide.
At that time Emanuele Andreano of the MAD Lab was working on isolating thousands of human monoclonal antibodies, including MAD0004J08, one of the most potent in evaluation for the treatment of COVID-19. “It was extremely effective,” he says. “The unfortunate thing was that in November 2021, the Omicron variant came and basically hit all the developers of monoclonal antibodies.”
Through evolution and mutation, the virus had escaped. Just weeks after announcing purchase orders for COVID-19 monoclonals, the U.S. government abandoned them in early 2022 for lack of effectiveness against what had then become the dominant Omicron strain. Some 3.35 million monoclonal antibody courses had been administered in the U.S. at that point, compared with 214 million vaccines in the country, and 4.1 billion immunizations worldwide.
In addition to viral escape, the manufacturing and delivery of antibodies also complicates their widespread use. Monoclonals are delivered either through a series of intramuscular injections — REGEN-COV was given in a course of four shots, two in the stomach and two in the arm — or through intravenous infusion, as for chemotherapy.
One advantage of antibodies is that they are easier to isolate and develop than small molecule drugs or vaccines. They are also effective within minutes to hours after administration instead of the weeks or months it can take to develop an immune response post vaccination. In the event of a pandemic, monoclonal antibodies can also be made quickly. But the downside is intravenous infusions and intramuscular injections can be more difficult to administer in mass quantities or in more remote geographies.
Manufacturing expenses and high costs are also significant obstacles to widespread use of antibody-based treatment and prevention. Even after COVID-19, there is still a lack of incentive for industrial investment in research and development and eventual production of monoclonals, particularly for use in low- and middle-income countries, as outlined over a two-day meeting in Geneva in March 2023 convened by Unitaid, IAVI, Wellcome, and the Medicines Patent Pool. As COVID-19 plainly illustrated, access to antibodies was largely limited to wealthy countries and therefore delivery and distribution of future products will require new business models.
And yet monoclonals define an avenue of investigation that has drawn a lot of attention, hope, and resources, especially within the HIV field. The field was buoyed by the discovery of numerous broad and potent neutralizing antibodies against HIV and quickly set to developing them as potential therapeutic and prevention options with the largest trials testing antibodies for HIV prevention, the Antibody Mediated Prevention or AMP trials, culminating in 2021.
The AMP results set a very high bar for the quantity and specificity of monoclonal antibodies that will be required to protect against the immense diversity of HIV strains in circulation today. Researchers speculate that three antibodies of different specificities, present at high concentrations, may be what it ultimately takes to ward of the virus with antibody prophylaxis. Post-AMP, the mountain to climb to develop and deliver effective monoclonal antibodies for HIV prevention became very steep.
“We’re not where we wanted to be,” says Carl Dieffenbach, director of the Division of AIDS at the U.S. National Institute of Allergy and Infectious Diseases. “We thought we had good antibodies, but, one by one, each has failed in different ways.”
Still, researchers aren’t abandoning HIV antibody-based treatment or prevention research. “We’re addressing a number of different questions by continuing to pursue using broadly neutralizing antibodies,” adds Dieffenbach. Researchers continue to test antibody therapeutics — at least three early trials of different monoclonal antibodies were presented at the recent Conference on Retroviruses and Opportunistic Infections (CROI) 2024 meeting. Antibody research also sheds light on HIV vaccine development and may be a component of an effective cure strategy.
But HIV-specific monoclonal antibodies may also be somewhat superseded by advances in long-acting antiretrovirals that are applicable for both treatment and prevention and may be available by injection or pill. As a result, antibody-based prevention may be confined to more strategic use or in specific, discrete at-risk populations.
“I think we have to remember what antibodies are good at,” says IAVI’s Jon Heinrichs, who leads the organization’s antibody program strategy and translational immunology function.
Heinrichs joined IAVI in 2023 from Sanofi, where among other things he supported the firm’s development of a monoclonal antibody protecting infants against respiratory syncytial virus (RSV). This virus causes a lower respiratory tract infection that can seriously harm infants up through 24 months, though it can affect all age groups. One intramuscular dose of Beyfortus, Sanofi’s trade name for its RSV antibody treatment, can protect an infant through an RSV season.
This is one setting that seems well suited for antibody development. “What they are really nice for is a couple of things: for individuals who cannot develop their own immune response, for example young or premature infants, or those on chemotherapy or in immunocompromised states,” Heinrichs says. “Or for people who are at risk for a defined period of time.”
One could imagine this for essential or public health care workers who are spending time in a danger zone. “If you have an outbreak situation where you need to deliver an immune response very rapidly, antibodies are a great solution,” he says.
Another potential application is blocking mother-to-child transmission of HIV during birth and through the breastfeeding period. An IAVI antibody, ePGT121v1.LS, is set for studies alone and in combination with two other antibodies, VRC07.523LS and PGDM1400LS, for potential use in infants to prevent mother-to-child transmission of HIV. These trials are expected to start in 2025. These ‘LS’ antibodies are engineered to have a higher potency and a longer half-life — potentially allowing for fewer and lower doses of antibody overall.
Lower doses could help offset the high cost of antibody manufacturing. Doses of COVID-19 monoclonals were set at about $2,000 each, but, as Heinrichs says, dosage for these therapies can be by weight, which highlights another advantage of delivering them to infants.
As COVID-19 showed so bluntly, for antibodies to be useful in infectious diseases they will also need to be targeted more precisely toward highly conserved regions on a pathogen. This is something HIV researchers are acutely aware of, but during COVID-19 developers had an emergency response and went after the most effective antibody they could engineer at the time, not the one that might retain coverage over multiple strains and mutations. “We went after the virus, and when the virus changed, we went after it again. But we were always following the virus,” Rappuoli says. “You have to get ahead of it. To go after regions where it cannot change or where it is extremely difficult for it to mutate.”
Rappuoli, Andreano, and a group of the MAD Laboratory researchers are taking this into consideration as they work to develop antibodies for a wide variety of pathogens, including drug-resistant bacteria. Andreano and his colleagues in Siena are interested in potential applications of monoclonal antibodies for tuberculosis and pneumococcal disease. Researchers in the lab recently isolated monoclonal antibodies against pandrug-resistant Klebsiella pneumoniae. They are also pursuing development of potential cross-protecting antigens derived from meningococcal vaccinees to be used against the antibiotic-resistant bacteria that causes gonorrhea.
Pursuing antibacterial antibodies presents many challenges — these antibodies are difficult to test and the bacterial pathogens present multiple antigenic targets on their cell surfaces, therefore binding to a single molecule may not be enough to counter them. Bacterial pathogens also present multiple strains.
“I don’t want to be too optimistic, but I don’t want to be too pessimistic,” Rappuoli says. “With staph, it’s never worked. You can say, well, these things fail. On the other hand, if you look back, these were antibodies developed 10 or 15 years ago with the technologies that were available. Now we can do 100,000 times better than we could do at the time.”
It might also be useful recalling that mRNA vaccines were seen once as long shots.
For further reading
- Antibody therapeutics approved or in regulatory review in the EU or US: https://www.antibodysociety.org/resourc ... ntibodies/
- Toscana Life Sciences: https://www.toscanalifesciences.org/en/ ... -projects/
- MAD Laboratory: https://www.toscanalifesciences.org/en/ ... y-mad-lab/
- GSK and Vir Biotechnology’s Phase III results for its COVID monoclonal, June 2021: https://www.gsk.com/en-gb/media/press-r ... otrovimab/
- An ongoing clinical trial for SARS-CoV-2 monoclonal therapy for immunocompromised people: https://pilotfeasibilitystudies.biomedc ... 23-01325-y